Renewable energy sources are becoming increasingly popular. However, such energy can be wasted if an excess is available when it’s not yet needed. A particularly relevant example is solar power; solar panels provide most of their output during the day, while often a household’s greatest energy use is at night.
One way to get around this problem is by storing excess energy so that it can be used later. The most common way this is done is with large batteries, however, it’s not the only game in town. Phase change materials are proving to be a useful tool to store excess energy and recover it later – storing energy not as electricity, but as heat. Let’s take a look at how the technology works, and some of its most useful applications.
It’s All About Heat
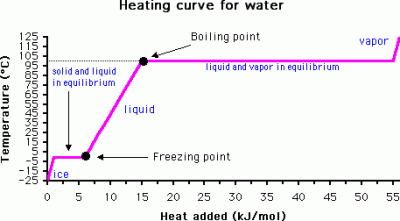
Unlike batteries or capacitors, phase change materials don’t store energy as electricity, but heat. This is done by using the unique physical properties of phase changes – in the case of a material transitioning between solid and liquid phases, or liquid and gas. When heat energy is applied to a material, such as water, the temperature increases. However, when the liquid water reaches temperatures close to boiling point, something strange happens.
As more energy is put in, the temperature begins to flatline. This is because enough energy must be put in to overcome what is called the latent heat of vaporization – the energy required to turn the liquid into a gas. Eventually, once enough heat is put in, the water turns to steam and the temperature is again free to rise. This latent heat can store a significant amount of energy in a material over a relatively small temperature change. This latent heat exists in solid-to-liquid phase changes as well, where it’s known as the latent heat of fusion. By taking advantage of latent heat, large amounts of energy can be stored in a relatively small change in actual temperature, and accessed by manipulating the phase change of a material.
Perhaps the most common form of phase change heat storage on the market is the sodium-acetate handwarmer. These handwarmers contain a sodium-acetate gel in a plastic pouch. When the gel is given a nucleation point by tweaking a metal disk in the gel, it quickly changes phase from a super-saturated liquid to a solid. Suddenly freezing like this releases the latent heat the material was holding in its liquid form, and warms the user’s hands nicely. The material can later be recharged by heating the handwarmer up to melt the sodium acetate once more, before allowing it to gently cool back down to room temperature. The latent heat will then be trapped in the liquid until it is once more disturbed, causing it to freeze again.
A wide variety of materials have been studied for heat storage through the phase change effect. Paraffin wax is perhaps one of the most commonly studied, thanks to its phase change occuring in a useful temperature range. However, its low thermal conductivity limits the rate at which energy can be exchanged, hampering performance. Hydrated salts have been another material of significant interest, though face problems of their own. Often, such materials will undergo subcooling. As heat is extracted from the liquid material, its temperature declines below freezing point without the material actually becoming solid. Without undergoing a change in phase, the latent heat remains trapped in the liquid, and can’t be extracted. Additionally, like many battery chemistries, repeated cycling can cause problems. The phase change material must retain its properties over many cycles, without chemicals falling out of solution or corrosion harming the material or its enclosure over time. Much research into phase change energy storage is centered around refining solutions and using additives and other techniques to engineer around these basic challenges. Often, the specifics of such materials remains a commercial secret as companies attempt to recoup research costs through sales.

The phase change effect can be used in a variety of ways to functionally store and save energy. Heat can be applied to a phase-change material, melting it and thus storing energy within it as latent heat. Excess electrical energy, such as from renewable sources, can readily be stored in such phase change materials, as it’s possible to turn electrical energy into heat quite efficiently. The reverse is not so easy, however.
Instead, such phase change devices are often instead used to output heat more directly – either by being used as hot water heaters or to supply heat energy to refrigeration processes. This is achieved often by simply passing working fluid, like water or refrigerant, through a heat exchanger in contact with the phase change material. The former has plenty of applicability to households, cutting down on costs for residential heating and hot water. The latter is of more relevance to large commercial and industrial facilities. Particularly in industries such as winemaking and cold storage, refrigeration can be a major bottom-line expense that is essential to operations. Even small percentage gains in efficiency or reduced energy use can have huge payoffs over time.

Another interesting use of phase-change materials is as a passive heat management solution for buildings. The idea is to use a phase change material with a melting point around a comfortable room temperature – such as 20-25 degrees Celsius. The material is encapsulated in plastic matting, and can be installed in a building in walls and ceilings along with insulation. The material then acts as a sort of thermal buffer. Heat energy building up in a room can be absorbed by the phase change material, keeping temperatures lower. As the building then cools, the material can release its heat, acting to stabilize temperatures. It can be a lightweight way to increase the thermal mass of a building, and can reduce the reliance on active cooling or heating from HVAC systems.

Looking to the future, it may be that phase change energy storage remains of limited use in the residential space. While it can have benefits, its limited heating-only application makes it less attractive than battery storage that can run an entire home. However, for industrial processes, such as refrigeration and process heating, there’s plenty of scope for phase change technologies to be used as a cheap and effective store of energy. With research ongoing in the field, it’s likely we’ll see greater uptake of this technology in future as energy conservation increases in relevance in future years.
No comments:
Post a Comment