Many years ago, I took a summer trip to the Maryland shore with some friends. One of my buddies and I got bored with playing football on the beach, so we decided to take a hike on one of the many trails back into the wooded area behind the dunes. At the trailhead we noticed a prominent sign, warning about the presence of “very aggressive mosquitos” and not to enter without first applying ample insect repellent. We scoffed at the warning as only young idiots could and soldiered on, bare-legged and confident that we’d be fine.
About three minutes into our hike, a small group came pelting down the trail in a panic. “It’s true! Turn back!” they shouted as they flew past us. Undeterred, or at least unwilling to appear that way to each other, we pressed on, only to discover a few minutes later that we were making a substantial blood sacrifice to the next generation of mosquitos on Assateague Island. We couldn’t bear more than a few seconds before turning tail and running back to the beach and jumping into the ocean to get rid of the last few dozen bloodsuckers.
I learned a valuable lesson from that experience, as well as developing a deep and abiding hatred of mosquitos. It turns out I’m in good company — pretty much everyone hates mosquitos, which are not just a nuisance but can be downright dangerous to be around. But if tests with genetically engineered mosquitos currently underway in Florida turn out well, we may be able to finally turn the tide against mosquito-borne diseases, simply by killing all the females before they ever reach adulthood.
The Circle of Death
To call mosquitos a scourge to humankind is perhaps underselling their true health impact over the millennia. The world’s 3,500-odd species of mosquitos are notorious for their ability to pick up a deadly payload of viruses, protozoans, and even worms when they feast on the blood of warm-blooded creatures, potentially transmitting them to the next hapless creature that comes along. It is estimated that there are over 700 million cases of mosquito-borne diseases every year, resulting in a million or more deaths. A single mosquito-borne disease, malaria, has been such a prolific killer throughout history that it could potentially have killed nearly half of the 110 billion or so humans that have ever lived.
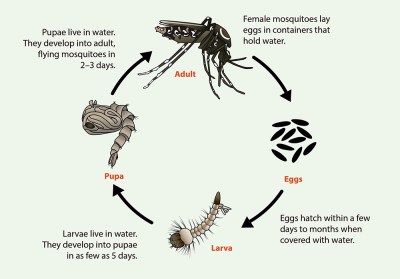
Mosquitos begin life as eggs laid in or near stagnant water. Dry eggs can survive for months; once exposed to water, they hatch within a few days. During their larval stage, mosquitos live and feed in the water. Within a week, the larvae enter the pupal stage, hanging just below the surface of the water as they develop into adults. The full-grown adults emerge two or three days later as they take wing and seek food and mates.
Both male and female mosquitos consume nectar from flowers and fruit, using the sugars for energy. The females, however, require a concentrated source of protein to produce a clutch of eggs, so after mating, they seek out a victim for a blood meal. Once the prey is located, the mosquito uses her mouthparts to saw into the flesh while secreting an anticoagulant in her saliva; it’s this exchange of fluids that carries the potential of transferring disease between parasite and host. A female may take several blood meals before accumulating enough nutrients for a clutch of eggs, which she lays in or near stagnant water before dying.
The semi-aquatic life cycle of most mosquitos seems like it would be the key to control of the insects and thereby, reduction of the diseases they spread. And indeed, a lot of the efforts of mosquito control projects over the last few hundred years have concentrated on eliminating or reducing mosquito-friendly habitats, by draining swamps and eliminating water catchments like tire dumps. Biocontrol using larvae-eating fish or introduction of bacterial spores that kill larvae have been tried, as have a host of pesticides, traps, and physical barriers, like thin films of non-toxic oils that kill mosquito pupae.
New Approach
Mosquito control is basically a numbers game, stacked in their favor. Since each female lays 100 to 200 eggs in a clutch, in wet climates, mosquitos are simply too prolific to get ahead of using standard means. Coupled with collateral damage to the environment — draining wetlands carries potentially huge impacts on a wide range of species, as does the indiscriminate use of pesticides — the search for new control methods with less harmful ecological side-effects has led to research into genetic methods of reducing mosquito populations.
The idea of genetically engineering insects is nothing new. The fruit fly Drosophila melanogaster has had its genome extensively modified for over 100 years, first using standard mating and crossing techniques and later using transgenic methods to insert, delete, and edit genes. The result has been a wealth of knowledge about how the genetics of higher organisms work, as well as models for human diseases ranging from diabetes to Parkinson’s.
But in general, transgenic fruit flies are simply model organisms destined to live and die in the lab. The concept of building a genetically modified insect for release into the wild is fairly new. Oxitec, the company behind the planned releases of transgenic mosquitos in Florida, has been working on the genetic control of a range of pest insect species since it was founded in 2002. They are currently on their second generation of genetically modified Aedes aegypti mosquitos, which is the insect that will soon be tested in Florida.
The mosquito, dubbed OX5034, has been genetically engineered to be self-limiting. Both male and female OX5034 mosquitos carry a synthetic gene that is lethal only to females. The plan is to release OX5034 male mosquitos into a wild population where they’ll breed with unmodified females. These females will take a blood meal and lay eggs that carry the synthetic gene. Only the male eggs in the clutch will develop into adulthood; the females will all die during the larval and pupal stage, which will eventually reduce the number of blood meals taken and the potential for disease spread.
Positive Feedback Turns Deadly
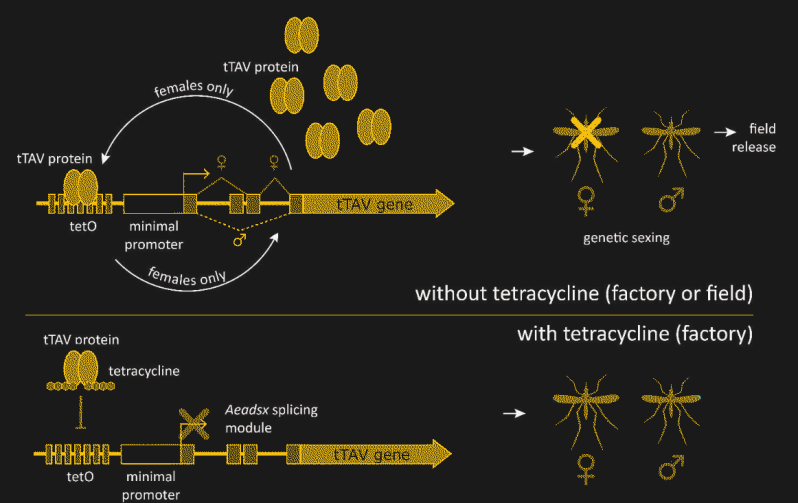
The details of how Oxitec has hacked the Ae. aegypti genome to achieve this level of control are pretty fascinating. The technique revolves around what’s known as a fusion protein, which takes pieces from two or more unrelated proteins and sticks them together into something new. In this case, the protein is called tTAV, or tetracycline-repressible transactivation. The tTAV fusion protein is essentially a nonsense protein, in that it doesn’t serve any useful biochemical function. But tTAV has a trick up its sleeve — it activates its own expression. Expression of the tTAV gene produces tTAV protein, which then binds to the gene to increase the production of tTAV. This positive feedback loop takes over the cellular protein production apparatus, filling the cell with useless tTAV protein and depleting the cell’s resources, killing the cell and eventually the organism.
The lethality of tTAV must be controlled, though, or it would be impossible to raise a population of insects that could survive to pass this trait along. That’s achieved by adding a small section of protein that binds tetracycline to tTAV. If the common antibiotic is present, it will bind to tTAV with a much higher affinity than tTAV binds to its own gene. That locks up the tTAV and keeps it from stimulating its own expression, breaking the positive feedback loop and allowing the insects to survive into adulthood. In the lab, tetracycline is added to the growth medium and food of OX5034 mosquitos, allowing the transgenic mosquitos to survive and breed.
There’s one more trait that has to be added to make this strategy work. To keep the modification active in the wild, tTAV lethal only to females. OX5034 relies on the fact that higher organisms often splice messenger RNA (mRNA) after it has been copied from genomic DNA. The spliced mRNA may have large sections of the original transcript edited out, or even have sequences from entirely different genes stitched together. The OX5034 gene has a sex-dependent splicing module added before the tTAV gene. In males, the OX5034 is spliced so that the gene for tTAV is never expressed, while in females, the tTAV protein is expressed in abundance, unless tetracycline is provided.
In the Field
Having created this controllable, female-specific lethal trait, Oxitec now plans to test it in the wild. The strategy is to produce a lot of OX5034 mosquitos in the lab in the presence of tetracycline so that the females survive. Once mated with the OX5034 males, the females get a meal of horse blood and lay their eggs. The eggs are collected, processed, and placed into special release boxes, which are deployed in the test locations. When activated with plain water, the release boxes act like incubators, allowing the eggs to hatch and the OX5034 mosquitos to start growing. In the absence of tetracycline, though, only the male eggs will progress past the larval stage.

The OX5034 males quickly mate with the native Ae. aegypti females in the test area, who take their blood meal and lay their clutch of eggs just like normal. However, her eggs will all carry a copy of the OX5034 gene, meaning that all the females in the brood will be dependent on tetracycline to survive, and die in its absence. The male eggs, also carriers of the gene, will survive to adulthood, carrying the lethal genetic payload forward to another generation of wild-type females. Tests have shown that OX5034 males are less hardy than wild-type mosquitos and disappear from the population within ten generations, which is about six months. Long-term control of Ae. aegypti would obviously then require a continuous introduction of OX5034 eggs into the environment.
As with any pest remediation project, the OX5034 approach has its risks. Oxitec’s plan for the release has been reviewed by multiple regulatory agencies, including the United State Environmental Protection Agency, which addressed many of the concerns related to the novel insects. One of the most obvious concerns is the presence of tetracycline in the environment. The EPA is therefore ordering Oxitec to not release any OX5034 mosquitos within 500 meters of potential tetracycline sources, including citrus groves, where the antibiotic is used to fight a bacterial disease known as citrus greening, or sewage treatment plants, which may contain tetracycline that has been excreted by humans. Five hundred meters might not seem like much, but field studies show that the vast majority of Ae. aegypti individuals are born, live, and die within a 30-meter radius, and in no case has a mosquito been trapped more than 200 meters from release.
Another potential problem is tampering. The release boxes look a little like plastic coolers and seem like they could be tampered with easily. The company addressed this concern in the Florida Keys test by enlisting only private property owners, mainly homeowners, to host release boxes. The tamper-evident boxes will mainly be secured in fenced yards and will be monitored regularly by Oxitec employees.
As can be expected, the Oxitec release has garnered considerable attention, and not all of it is favorable. This is a new technology, and releasing any transgenic organism into the wild is something that needs to be done with all due regard to safety. But if the current Oxitec tests pan out, genetically engineered insects may be just the leading edge of a wave in innovation in pest control and disease mitigation.
No comments:
Post a Comment